Face Mask
Quick Guide to Face Mask Selection and Use
The major difference between N95 medical and KN95, N95, FFP2 industrial models is N95 medical ones have protection for body fluid. For non surgical usage, you only need buy N95 industrial one.
Choose the right mask for the task! Select the mask design, fit and filtration that matches the protection needs for each procedure or risk level.
Filtration Level | Performance | Usage |
KN95, N95, FPP2 Surgical | MAXIMUM FILTRATION NIOSH Approved N95/equivalent standard KN95 Particulate Respirator High Fluid Resistance 160 mmHg Filtration Efficiency BFE >=99.8% PFE = 99.9% @ 0.1 micron Breathability -Delta P <5.0mm H2O/cm2 Flame Spread Class 1 | Indicated for use when treating patients with airborne diseases such as TB or influenza.* |
KN95, N95, FPP2 Industrial | KN95 Particulate Respirator Meets NIOSH 42 CFR 84 N95 requirements for a minimum 95% filtration efficiency against solid and liquid aerosols that do not contain oil. Breathability - Delta P <5.0mm H2O/cm2 Flame Spread Class 1 | To reduce risk of COVID-19 or influenza in non- surgical situation for all: police, retail, firefighter, etc. in hospital (except surgical), post office, commercial buildings, construction,food processing, food Safety, general manufacturing, heavy infrastructure, mining, oil & gas, transportation |
ASTM Level 3 | ASTM LEVEL 3 High Fluid Resistance 160 mmHg Filtration Efficiency BFE ≥ 98% PFE ≥ 98% @ 0.1 micron Breathability - Delta P <5.0mm H2O/cm2 Flame Spread Class 1 | Ideal for procedures where heavy to moderate amounts of fluid, spray and/or aerosols are produced. Leave these for medical professionals. |
ASTM Level 2 | ASTM LEVEL2 High Fluid Resistance 120 mmHg Filtration Efficiency BFE ≥ 98% PFE ≥ 98% @ 0.1 micron Breathability - Delta P <5.0mm H2O/cm2 Flame Spread Class 1 | Ideal for procedures where moderate to light amounts of fluid, spray and/or aerosols are produced. Leave these for medical professionals. |
ASTM Level 1 | ASTM LEVEL1 High Fluid Resistance 80 mmHg Filtration Efficiency BFE ≥ 95% PFE ≥ 95% @ 0.1 micron Breathability -Delta P <4.0mm H2O/cm2 Flame Spread Class 1 | Ideal for procedures where low amounts of fluid, spray and/or aerosols are produced. |
LOW PERFORMANCE | LOW PERFORMANCE Surgical Molded Utility Mask Physical Barrier Only Filtration Efficiency N/A | Ideal as a comfortable substitute for earloop face masks, this mask is a simple physical barrier ideal for exams and visitations or for dry, short procedures that do not produce fluid, spray or aerosols. |
We can help you purchase and import all kind of face masks for medical surgical purposes or general protection. We find you FDA registered, PPE Regulation (EU) 2016/425 CE certified face masks.
Purpose or Intended Use
It is easy to confuse a surgical mask, a surgical N95 (KN95/FFP2) respirator, and an industrial N95 (KN95/FFP2) disposable respirator with one another. They look similar, and the words "respirator" and "mask" are often used interchangeably when discussing respiratory protection. However, in fact there are many differences between them. This information here is intended to educate you on the differences between surgical masks, surgical N95 respirators, and industrial N95 respirators.
Surgical masks
- May include masks labeled as surgical, laser, isolation, dental, or medical procedure masks
- Are primarily intended to protect the patient, not the wearer, from the wearer's saliva and respiratory secretions
- May also help protect the wearer against exposure to microorganisms, body fluids, and large particles in the air but are not tight fitting and likely have substantial inward leakage for particles and organisms
- Are designed to cover the mouth and nose loosely but are not sized for individual fit
- Are used to help protect the environment and other nearby persons from the wearer's contaminants
- Are not NIOSH (National Institute for Occupational Safety and Health) approved
Surgical N95 respirators
- Surgical N95 respirators are designed to reduce but cannot eliminate the wearer’s exposure to airborne biological contaminants. They do not eliminate the risk of illness, disease, or death.
- Form a tight seal over the mouth and nose.
- Require fit-testing and must be adjusted to your face to provide the intended effectiveness of filtering 95 percent of particles with a mass median diameter of 0.3 micrometers.
- Employers and users are required to follow the OSHA Respiratory Protection Standard, 29CFR 1910.134, as well as other state or local regulations, as appropriate.
- Have specific use instructions, warnings, and limitations for use in health care environments.
- Are NIOSH certified.
- Are fluid resistant to a certified level measured against a stream of artificial blood directed at the respirator.
Industrial respirators (including industrial N95 respirators)
In the United States, NIOSH is responsible for testing and certifying respirators to be used in the workplace. NIOSH not only reviews the manufacturer's test data, but also performs its own independent tests on the respirators in NIOSH's governmental laboratories to verify the manufacturer's results. The tests include filter efficiency, degradation, and flow rate, to name a few. In addition to testing the respirators during the submittal process, NIOSH also will periodically purchase respirators in the field and test them to make sure the respirators are performing to their original certification.
Respirators are designed to seal the respirator to the face and pass a fit test. Under Respiratory Protection Standard 29 CFR 1910.134, the Occupational Safety and Health Administration requires the wearer of a respirator to be fit tested before he or she can use the respirator in a contaminated environment. OSHA also requires the wearer to perform user seal checks on the respirator before each use, as well as comply with the other elements of a comprehensive respiratory protection program in accordance with 29 CFR 1910.134.
Once the respirator is initially approved, NIOSH will certify its classification as N, R, or P and its filter efficiency as 95 percent, 99 percent, or 99.97 percent. It is also important to note that even though a respirator just by its use often helps to prevent the wearer from contaminating the environment; it cannot be considered a surgical mask unless it has been cleared by the FDA.
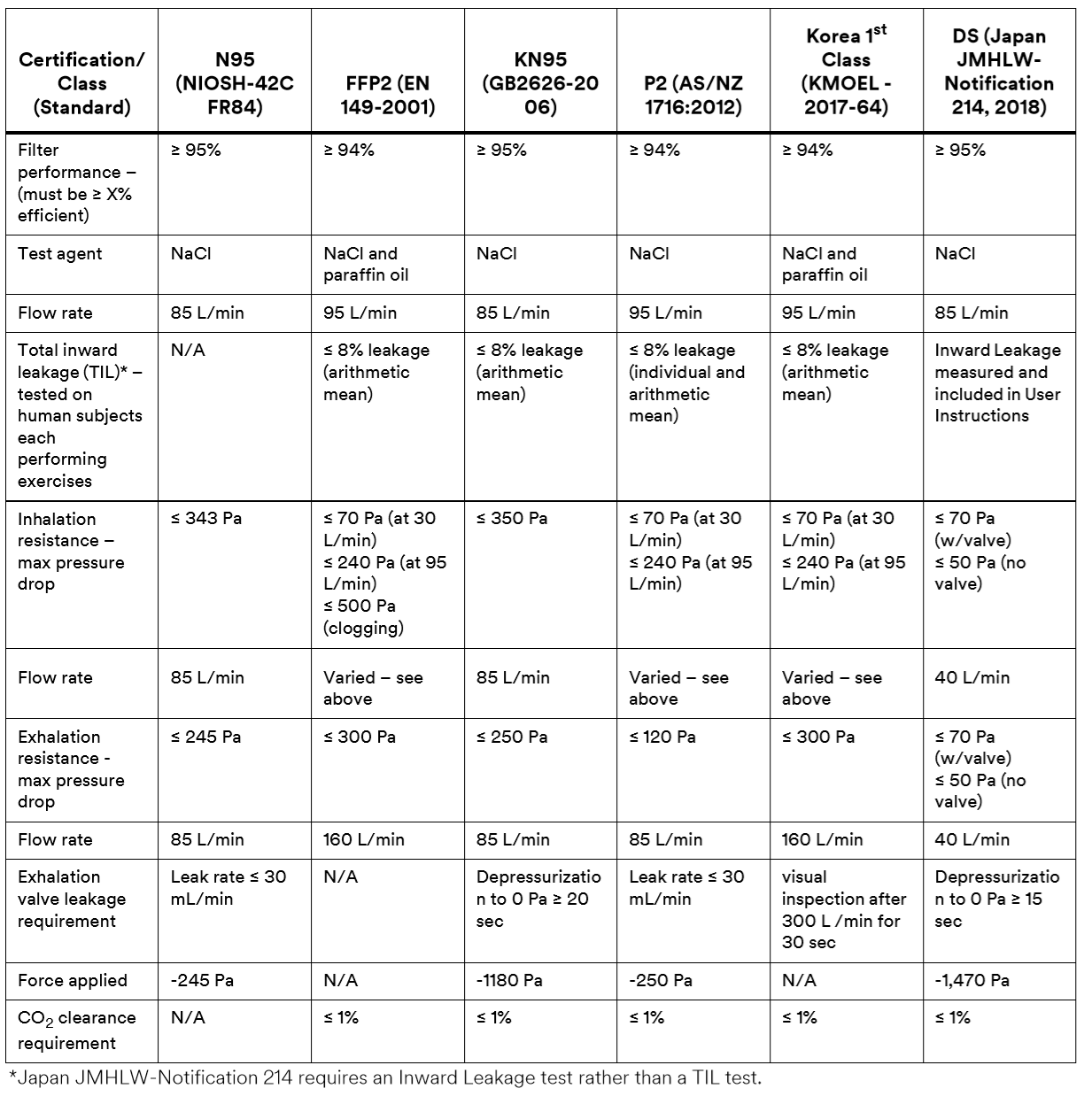
Major Regulation Standards around World
China KN95, AS/NZ P2, Korea 1st Class, and Japan DS FFRs are “equivalent” to US NIOSH N95 and European FFP2 respirators, for filtering non-oil-based particles such as those resulting from wildfires, PM 2.5 air pollution, volcanic eruptions, or bioaerosols (e.g. viruses).